Carbon can be arranged in a number of configurations. When each of its atoms is bonded to three other carbon atoms, it's relatively soft graphite. Add just one more bond and it becomes one of the hardest minerals known, diamond. Chuck 60 carbon atoms together in a soccerball shape and boom, buckyballs.
But a ring of carbon atoms, where each atom is bonded to just two others, and nothing else? That's eluded scientists for 50 years. Their best attempts have resulted in a gaseous carbon ring that quickly dissipated.
So it's a pretty big deal that a team of researchers, from Oxford University and IBM Research, has now created a stable carbon ring.
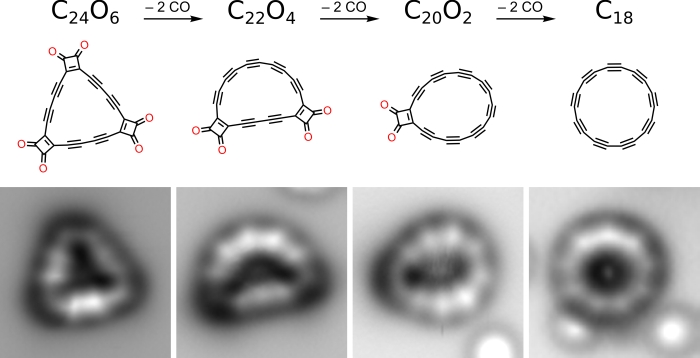
The ring-shaped carbon compound, called a cyclocarbon, is made from 18 carbon atoms, the smallest cyclocarbon that is predicted to be thermodynamically stable. And advanced microscopy techniques have provided images of it.
So far, research into the cyclocarbon's structure has suggested that it acts as a semiconductor, which means it has potential use in electronics. And the very property that made cyclocarbons so difficult to isolate in the first place - their high reactivity - means they could be used to create other carbon allotropes and carbon-rich materials.
To pull off this feat, the researchers started by synthesising the triangular cyclocarbon oxide C24O6. That's the 18 carbon atoms, bonded to six carbon monoxide molecules, two clustered at each of the three corners of the triangle.
They transferred this concoction to a layer of sodium chloride on a copper plate, chilled in a vacuum chamber to just above absolute zero. This provides an inert surface that keeps the structure stable.
Then, using the tip of an atomic force microscope, the team knocked the carbon monoxide (CO) molecules off the structure, leaving just the ring of carbon atoms behind.
No comments:
Post a Comment